
Corrosion is notorious as unmerciful enemy of the aircraft structure since the very inception of Aviation industry. A complex electro-chemical process that causes the metal to be transformed into their respective salts and oxides. These powdery substances replace the metal and cause severe loss of strength in the structure.
Principle
Basic principle of nature driving this phenomena is that everything in the universe tends to return to its origin. As already mentioned that it’s an electro-chemical process which makes metals and alloys to undergo a conversion into oxides, hydroxides and salts. In the overall process, two chemical reactions take place. One is Anodic reaction in which atoms of metal are ionized and are passed into their solution and electrons are left within original metal surface. In the other type of reaction that is Cathodic reaction, species such as O2 and H2O take up the free electrons available within the metal thus causing a reduction reaction.
Essential Requirements
Three basic necessities which are essentially required in order to form the corrosion are as under:
Corrosion Basic Types;
a. Oxidation Corrosion:
One of the more simple forms of corrosion and perhaps the one with which we are most familiar is ‘dry’ corrosion or it is most generally known as oxidation. When a metal is exposed to a gas containing oxygen, a chemical action takes place at the surface between the metal and gas. This action forms the oxidation corrosion.
b. Uniform Surface Corrosion:
When an area of unprotected metal is exposed to an atmosphere containing battery fumes, exhaust gases or industrial contaminants, there will be a uniform attack over the surface area. With this cause, corrosion takes place on entire surface. It is known as a uniform surface corrosion.
c. Pitting Corrosion:
A logical progression from uniform surface corrosion if left untreated is called pitting corrosion. This type of corrosion forms pits on the surface of the metal.

Figure 1 : Pitting Corrosion
d. Intergranular Corrosion:
This type of corrosion takes place due to improper heat treatment of metals. It attacks on the boundaries of a metal. It is most common type of corrosion on alloys and is very difficult to detect unless minute cracks are visible in the critical stages.
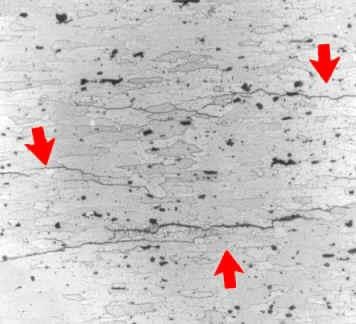
Figure 2 : Intergranular Corrosion
e. Galvanic Corrosion:
This type of corrosion occurs any time when following two conditions are met.
(1) Two dissimilar metals are connected in such a way that it provides a path for electron flow.
(2) Their common surfaces are covered with some material to serve as an electrolyte.

Figure 3 : Example of Galvanic Corrosion
f. Stress Corrosion:
The type of corrosion occurs due to presence of tensile stresses. A common place for stress corrosion is between rivets in a stressed skin, around bushings or tapered pipe-fittings. It can be detected by Dye-Penetrate Inspection method.

Figure 4 : Stress Corrosion
g. Fretting Corrosion:
This type of corrosion occurs when two surfaces fit tightly together but can move relative to one another. An un-wanted play between two metallic surfaces can lead to this type of corrosion.
Figure 5: Fretting Corrosion
Figure 6 : Basic Types of Corrosion
Causes of Corrosion;
a. Acid and Alkalis:
For corrosion to be formed on a metal surface there must be an electrode potential difference and an electrolyte. Almost all acids and alkalis react with metals to form corrosion.
b. Salts:
Marine atmosphere and air above some of the industrial areas hold a large concentration of salts. These chemicals will precipitate out of the air and may settle down on any of the metallic surface. These chemicals can cause formation of corrosion.
c. Mercury:
Mercury attacks aluminium by a chemical reaction known as amalgamation. In this process, the mercury rapidly attacks along the grain boundaries of the aluminium and corrosion occurs.
d. Water:
Pure water will react with metals to cause corrosion or oxidation but water holding a concentration of salts or other contaminants will cause much more rapid corrosion. Sea planes are continually in a battle with this element and every precaution must be taken to stay ahead of the formation of corrosion. Sea plane operation in salt water is especially vulnerable to attack. When a seaplane is taken out of the water, proper treatment is to be taken care of.
e. Air:
Naturally, it is impossible to isolate the structure of an aircraft from the air in which it exists. Air borne salts and other chemical compounds settle into the surface of an airplane and attract moisture from surrounding air. Corrosion then has the key pre-requisite that is an electrolyte.
Figure 7 : Typical causes of corrosion
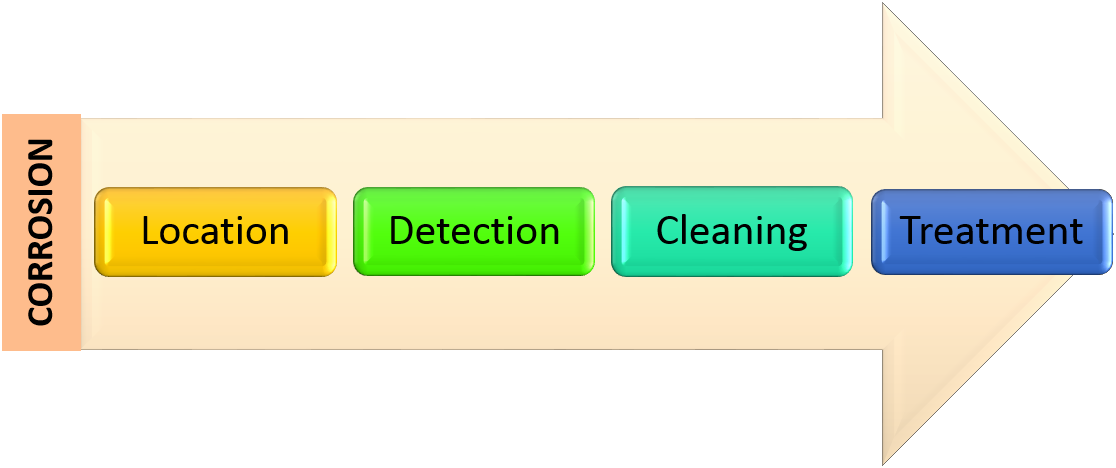
Corrosion Control;
It can safely be said that corrosion cannot be prevented absolutely but it can be controlled by eliminating one or more of the basic requirement for its formation, like:
There are certain basic operations which can be used to accomplish the above mentioned requirements for eliminating the favourable conditions leading to the corrosion, shown in the figure. Apart from this, step by step procedure of controlling Corrosion on aircrafts is mentioned in succeeding paragraphs.
(a)
(b)
Figure8 (a) (b) :Corrosion Control Process
a. Locating the Corrosion:
First and foremost step in controlling the corrosion in aircrafts involves the finding of exact location of affected area. Most probable areas which are considered highly susceptible to fall prey to this inherent enemy of metals are mentioned as under. These areas should frequently be inspected for any signs of corrosion to timely and effectively take counter measures accordingly.
b. Detection and Monitoring:
Corrosion monitoring and detection techniques involve a wide range of techniques. Various Non Destructive testing methods are most effectively and widely being used all over the world. Some of these methods are mentioned as under:
c. Cleaning Process:
After finding the corrosion and its proper detection and monitoring process, the most important step for corrosion control is a thorough cleaning of the affected area of the airplane. An emulsion-type cleaner that meets MIL-C-25769 specification does an excellent job of removing grime, dirt, exhaust residue, and dried oil and grease deposits.
d. Treatment Process:
Process of treating the corrosion varies according to the type of metal affected by this process. It is a comprehensive topic itself and subsequently needs detailed elaboration. However, some basic guidelines are presented below to treat corrosion on different types of metal surfaces on an aircraft. Mostly used alloys or metals including Aluminium Alloy, Ferrous Metals and Magnesium Alloys are discussed in following paragraphs.
A) Aluminium Alloy;
a. Mechanical Corrosion Removal:
After removing all of the paint and all traces of corrosion products, the white powder must be removed from the surface. Very mild corrosion may be removed by using a household abrasive cleaner such as Bon-Ami. Be sure that the abrasive does not contain chlorine. Nylon scrubbers such as Scotch-Brite can be used to remove this mild corrosion. Brushing with aluminium wool or an aluminium wire brush can remove more severe but not heavy corrosion.
b. Chemical Neutralization:
Remove all of the corrosion from the affected metal of the aircraft, then surface should be treated with a 5% chromic acid solution to neutralize any remaining corrosion salts. After this, acid is applied on to the affected surface for at least five minutes. It should be washed off with water and allowed to dry. Alodine treatment conforming to MIL-C-5541 will neutralize the corrosion as well as forming a protective film on the surface of the metal.
c. Protective Coating:
1) Cladding. Pure aluminium is considered to be non- corrosive. This is not altogether true because aluminium readily acts with the oxygen in the air to form air oxide film.
2) Surface Oxide Film. The characteristic of aluminium to form an oxide film on its surface, a film that resists further oxidation, is of real value in protecting the aluminium from corrosion. Metallurgists have found ways of forming films on metal surfaces that are hard, decorative, waterproof and airtight. There are two ways in which this film may be formed. It is most generally done by a chemical process known as Anodizing.
d. Electrolytic Treatment:
In the anodizing process, the part to be treated is cleaned in a hot water bath with a special non-caustic soap solution and then made the anode in an electrolytic process. Chromic acid and water form the electrolyte. After the formation of the oxide film, the part is washed in hot water and air-dried. Aluminium treated by this process has not been affected appreciably. In regard to its tensile strength, its weight or its dimensions. The anodic film on aluminium alloy is normally a light gray color varying to a darker gray for some of the alloys. Some aluminium alloy parts, such as fluid line fittings of aircrafts are dyed for identification. This dye is applied in the anodizing process where it colours the oxide.
e. Chemical Treatment:
When small parts are fabricated in the field or where the protective anodizing film has been removed, the part may have a protective film applied by chemical rather than electrolytic methods. This process uses a chemical known as Alodine 1201 or if an invisible film is desired, Alodine1001 is used. These preparations are proprietary products, which meet MIL-C-5541 specifications. Alodine can be applied to a surface that has all traces of corrosion mechanically removed.
f. Organic Film:
One of the most universally used corrosion control devices for metal surfaces is a good coat of paint. On porous surfaces, paint adhesive is not a problem. But on some metal surfaces, it may be difficult to get the paint to stick. The surface of aluminium must be prepared in order to ensure that the paint will have a rough surface on which it has to adhere. The surface may be roughened up with a mild chromic acid etch or by either anodizing or Alodining. All of these treatments provide a good base for the primer to which the paint film will adhere.
B. Ferrous Metals;
1. Mechanical Corrosion Removal:
a. Unlike aluminium, the oxide film that forms on ferrous metals is porous and will attract moisture and continue to convert the metal into corrosion or rust. All traces of this rust must be removed from the aircraft forthwith. Most effective method of removing it is by mechanical means. Abrasive papers, wire brushes, both hand and power driven. Sand, aluminium oxide or glass beads may be used effectively as well. If a part has been plated, either with Cadmium or Chromium, care must be taken to protect the plating as it is usually impossible to restore it in the field.
b. Highly stressed steel parts such as found in landing gears must be cleaned with extreme care. If corrosion is found on these parts, it should be removed immediately by removing the absolute minimum amount of material. A fine stone, fine abrasive paper or pumice may be used. Wire brushes should not be used as they cause minute scratches which promote stress concentrations and potentially weaken the part.
c. If abrasive blasting is used, it must be done with caution by using only very small grit abrasive or glass beads. After all of the corrosion has been removed, the rough edges of any pits or damage must be faired with a fine stone or 400 grit abrasive paper and the surface primed as soon as possible. A dry, clean surface is an ideal setting for new corrosion and if not protected immediately, it will allow new damage to begin. Zinc chromate primer may be used on most freshly cleaned steel surfaces.
2. Surface Treatment:
a. Nickel or Chrome Plating:
This electroplated coating of nickel or chromium is used to form an airtight coating over the metal to exclude air and moisture from the base metal. There are two types of chrome plating used in aircraft maintenance. Decorative Chrome is used primarily for its appearance and surface protection but Hard Chrome is used for wear resistance in piston rods, cylinder walls, and other parts subject to abrasion. Parts using hard chrome are normally undersize and plated to the proper dimensions. Engine cylinder walls are normally plated with porous chrome whose surface has thousands of tiny cracks, which hold lubrication.
b. Cadmium Plating:
While chrome plating protects steel by excluding moisture from its surface, cadmium plating protects it in an entirely different way, by sacrificial corrosion. Almost all steel hardware of aircraft is cadmium plated. This soft silvery-gray metal is electroplated on the steel parts to a minimum thickness of 0.005 inch. It provides an attractive finish as well as protection against corrosion. When the cadmium plating on a part is scratched through to the steel, galvanic action takes place in which the cadmium corrodes. If the steel should corrode, action could continue until the part is ruined, but the oxides which form on the surface of cadmium are similar to those which form on Aluminium; that they are dense, airtight and watertight. No further action takes place. Once the initial film has formed. This type of protection is known as sacrificial corrosion.
c. Galvanizing:
Some steel parts such as firewalls are treated with a coating of zinc. The protection afforded by galvanizing is similar to that provided by cadmium plating: it is also sacrificial corrosion. When scratched through, zinc will corrode and provide airtight oxide film .The zinc is applied by passing steel sheets through vats of molten zinc, then rolling them through a series of rolling.
d. Metal Spraying:
Aircraft engine cylinders are sometime protected from corrosion by spraying molten aluminium in their surface. The steel cylinder barrel is sandblasted absolutely clean and dry. Aluminium wire is fed into an acetylene flame and high-pressure compressed air blows the molten aluminium onto the surface. Corrosion protection afforded by this treatment is sacrificial corrosion such as is provided by cadmium or zinc coating.
e. Organic Coating:
Since paint is an effective and widely used corrosion protection system, its film must not be allowed to break down. The surface to be painted must be properly prepared. Dry abrasive blasting will remove all surface oxides and roughen the surface enough to provide a geed bond for the paint. Parts which have been cadmium plated normally must have their surface etched with a five percent solution of chromic acid before the primer will adhere. After a clean, dry surface has been prepared, a thin,
Wet coat of zinc chromate primer is sprayed on and allowed to dry. The final finish can usually be applied after about an hour.
C. Magnesium Alloys;
a. Mechanical Corrosion Removal. Magnesium is one of the most active of the commonly used metals for aircraft construction but its weight to strength ratio is such that designers will accept its corrosiveness. A magnesium alloy does not naturally form a protective film on its surface, the way aluminium does, so special care must be taken that the chemically or Electrolytically deposited film is not destroyed. Magnesium corrosion occupies a larger volume than the pure metal so it will raise the paint or if it forms between skins, will swell the joints.
b. When corrosion is found on a magnesium structure, all traces must be removed and the surface treated to inhibit further corrosion. Since magnesium is anodic to almost all of the commonly used aircraft structural metals, the removal of corrosion cannot be done with metallic tools. They will leave contaminants imbedded in the metal and cause further damage. Stiff non-metallic brushes or nylon scrubber may be used to remove corrosion from the surface or from pits. Deep pots must be cut out with sharp steel or carbide tipped cutting tools or scrapers.
c. Carborundum wheel or paper must not be used because of its tendency to contaminate the surface and cause galvanic corrosion. If abrasive blasting is used, use only glass beads, which have been used for nothing but magnesium. Scale left in the abrasive form other metals can be imbedded in the magnesium and further damage occurs.
d. Surface Treatment. After all of the corrosion possible has been removed from the surface, a chromic acid pickling solution which conforms to MIL-M-3171A Type 1 (Dow No 1) should be applied to neutralize any corrosion products left. A satisfactory substitute solution may be made by adding about fifty drops of battery acid (sulfuric acid) to a gallon of ten percent chromic acid solution. Apply this to the surface with rags and let it stay for about ten or fifteen minutes; then rinse thoroughly with hot water. A treatment which forms a more protective film is a dichromate conversion treatment, Dow No. 7, which conforms to MIL- M-3171A Type IV. This solution is applied to the metal and allowed to stand until an oxide film of golden brown appears uniformly on the surface.
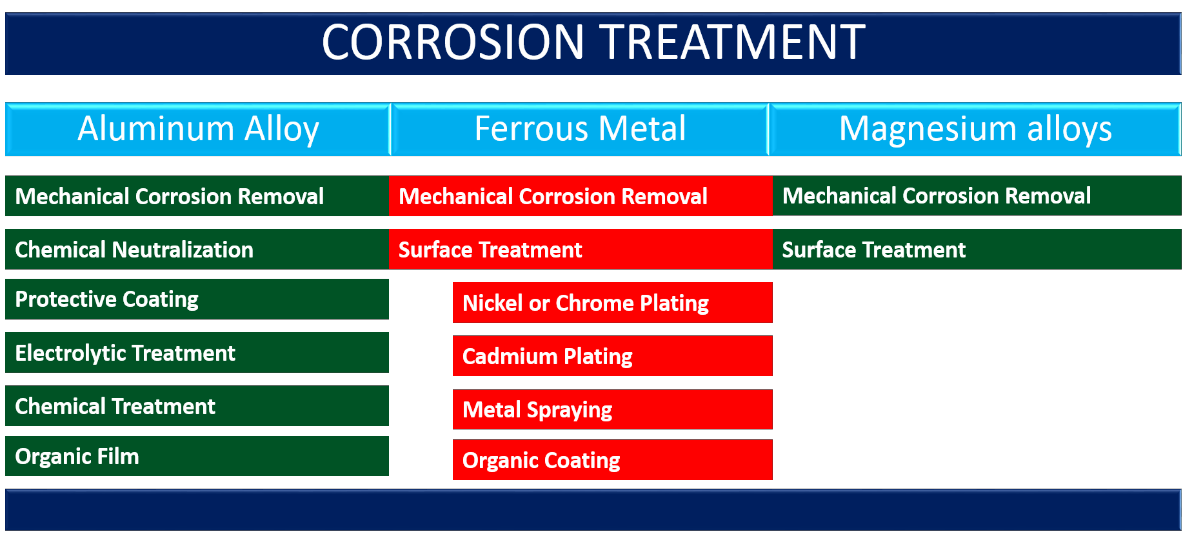
Figure 9 : Corrosion Treatment of various metals